Title/Area of PhD Research
Supervisory Team :
Dr Ahmed M. Eissa (Senior Lecturer); Dr Leigh Jones (Senior Lecturer); Professor Iza Radecka (Chair)
About the Project:
The aim of this project is to develop synthetic multivalent ligand systems based on glycosylated polymersomes with tunable rigidity, permeability and size as a simple mimic of biological cells and a new delivery system for bioactive molecules.
Polymer vesicles (polymersomes) are spherical soft-matter (nano)capsules consisting of a bilayer membrane enclosing an aqueous compartment and are generally formed by spontaneous self-organisation from amphiphilic block copolymers. Compared to lipid vesicles (liposomes), they have a relatively thick and robust membrane formed by polymeric amphiphiles with a relatively high molecular weight, which can increase their biological stability and prolong the circulation time in blood. Furthermore, polymersomes can present biologically active functionalities, such as sugars, on their external surface by self-assembly of functionalised amphiphilic polymers. Many biological processes in mammalian cells, such as fertilisation, viral and microbial infections, inflammation and cancer cell metastasis, are mediated by sugar-protein (lectin) interactions. Multivalent glycosylated macromolecules (glycopolymers) bind to lectins with high avidity and so provide an attractive therapeutic strategy for tackling diseases that involve sugar-lectin binding during disease progression. Moreover, these sugar molecules through interaction with cell-surface lectins can promote uptake of nanoscale particles by cells (e.g. galactose binds specifically to liver cells that possess high levels of the receptor ASGP-R). Sugar-decorated polymersomes (glycopolymersomes) therefore hold great promise in nanomedicine as vectors for targeted delivery and a simple model of biological cells.
In this project, we will synthesise well-defined amphiphilic block glycopolymers using the recently developed photo-induced reversible deactivation radical polymerisation and study their self-assembly, by different methods, into both small and giant unilamellar vesicles. Molecular characterisation will be performed by NMR, MALDI-tof MS and GPC, and extensive studies of the aqueous solution behaviour of these polymers (solubilities, cloud point, critical aggregation concentration, dynamic light scattering (DLS) and transmission electron microscopy (TEM)) will also be performed. Loading of the vesicles with model compounds and therapeutics, as well as studies of the membrane permeability will be explored.
This project is part of ongoing research, so is suitable for either Chemistry or Biomedical Sciences PhD students as the direction of work can be adapted and shifted from one aspect to another with support from the team.
Relevant recent references:
- Y. Li, Y. Chang, D. M. Haddleton, N. R. Cameron and A. M. Eissa “Comprehensive Glycoscience 2nd edition, Volume 4: Glyconanotechnology, Chapter 00114. Glycopolymer Functionalized Nanoparticles and Their Applications” by the Elsevier 2021, 209-249.
- A. R. Hall, J. T. Blakeman, A. M. Eissa, et al., “Glycan–glycan interactions determine Leishmania attachment to the midgut of permissive sand fly vectors”, Chemical Science 2020,11, 10973-10983.
- L. Martin, et al., “Polydimethylsiloxane-Based Giant Glycosylated Polymersomes with Tunable Bacterial Affinity”, Biomacromolecules 2019, 20, 3, 1297-1307.
- Y. Luo, et al., “Synthesis of glycopolymers with specificity for bacterial strains via bacteria-guided polymerization”, Chemical Science, 2019, 10, 5251-5257.
- J. Binfield, et al., “Imaging Proton Transport in Giant Vesicles through Cyclic Peptide–Polymer Conjugate Nanotube Transmembrane Ion Channels”, Macromolecular Rapid Communications 2018, doi.org/10.1002/marc.201700831.
- A. Kubilis, et al., “Giant polymersome protocells dock with virus particle mimics via multivalent glycan-lectin interactions”, Scientific Reports 2016, 6, 32414.
- A. M. Eissa, et al., “Glycosylated nanoparticles as efficient antimicrobial delivery agents”, Biomacromolecules 2016, 17, 8, 2672-2679.
- A. M. Eissa, et al., “Polymersome‐forming amphiphilic glycosylated polymers: Synthesis and characterization”, J. Polym. Sci. Polym. Chem. 2013, 51(24), 5184-5193.
For more information: For an informal discussion please contact via direct email to Dr Ahmed Eissa (A.M.Eissa@wlv.ac.uk)
Supervisory Team: Dr Leigh Jones (SL Chemistry) and Dr Ahmed Eissa (SL Chemistry)
About the Project:
Recent work emanating from the Jones group has described the synthesis of the novel ligand “divan” (LH2) that upon Cu(II) metalation forms the complex [(MeCN)ÌCu(II)2(L)2] (1; Fig. 1) [1]. The two singly deprotonated L- ligands in 1 twist away from one another to form the dimeric Cu(II) structure. The distorted square planar metal geometries exhibit long apical Cu-NMeCN interactions formed through the accommodation of a guest MeCN molecule to give pseudo square based pyramidal topologies at both metal sites. Moreover, the guest MeCN has formed a formal Cu-N-Cu magnetic pathway in 1. This PhD project will explore further the chemistry of this prototype molecule as described below in the form of two work packages (WP1-2).
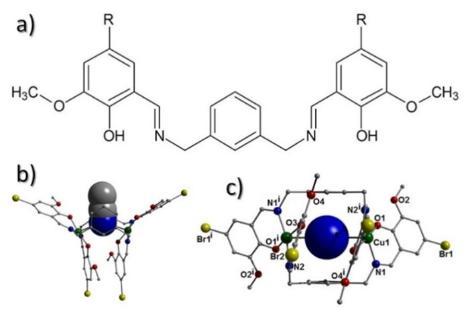
Figure 1 (a) Chemsketch of the ligand divan (LH2; R = Br). (b) Crystal structure of [(MeCN)ÌCu(II)2(L)2] as viewed perpendicular (b) and parallel (c) to the space-fill represented acetonitrile guest moiety. Colour code: Cu (green), C (grey), N (dark blue), O (red), Br (yellow). All hydrogen atoms have been omitted for clarity.
Work package 1: We will explore further the guest accommodating ability of this dimeric complex. Through careful guest selection we will be able to modulate and fine tune the resultant Cu(II)…Cu(II) magnetic exchange. Potential guests include (not exhaustive) pyrimidine (correct topology to forge 2 disparate Cu-Npyrimidine interactions); benzene / toluene (correct fit and would produce strong intermolecular interactions) and the azide (N3¯) anion can force ferromagnetic magnetic exchange. We will also investigate potential solution host-guest behaviour using NMR titration and / or UV-vis titration techniques [2].
Work package 2: Work would also focus on ligand modification towards molecular cavity modulation and therefore fine-tuning guest affinity with respect to the resultant host complex. Potential changes include Br replacement using Suzuki coupling transformations with (for instance) various commercially available boronic acid; [3] and the reduction of the imine C=N functional groups with NaBH4 or sodium triacetoxyborohydride (STAB) [4]. The initial change would potentially increase the molecular cavity size while the latter would significantly alter the ligand topology and would inevitably lead to a different complex topology upon metalation. In the same vein, we will also investigate the coordination ability of these novel ligands with other transition metals towards different magnetic behaviour. Likewise, diamagnetic Zn(II) analogues to 1 would be sought towards the aforementioned NMR titration studies.
The target material described here will be characterised via numerous techniques such as XRD (powder and single crystal); SQUID magnetometry and EPR (in conjunction with the University of Manchester). In summary, the successful execution of this project would give rise to a novel family of host-guest materials capable of hosting targeted guest molecules either towards their stabilization / sequestration or alternatively, fine-tuning magnetic exchange between the host magnetic metal centres.
References
1. L. F. Jones et al. Unpublished results.
2. P. Thordarson. Chem. Soc. Rev., 2011, 40, 1305-1323.
3. C. C. C. Johansson Seechurn, M. O. Kitchin, T. J. Colacot and V. Snieckus. Angew. Chem. Int. Ed., 2012, 51, 5062-5085.
4. M. B. Fugu, R. J. Ellaby, H. M. O’Connor, M. B. Pitak, W. Klooster, P. N. Horton, S. J. Coles, M. H. Al-mashhadani, I. F. Perepichka, E. K. Brechin, L. F. Jones. Dalton Trans., 2019, 48, 10180-10190.
For more information: For an informal discussion please contact via direct email to Dr Leigh Jones (Leigh.Jones@wlv.ac.uk)
Supervisory Team: Dr Ahmed Eissa (SL, Chemistry) and Dr Leigh Jones (SL, Chemistry)
About the Project:
The functionality, thermal stability, tuneable porosity and significant surface areas of both Metal-Organic Frameworks (MOFs)1 and Biopolymers2 has rendered them extremely promising hosts for the encapsulation (and immobilisation) of enzymes, thus allowing their use outside of the cell (Cell Free Enzymatic Catalysis). This project will combine the strengths of the PIs (Dr Leigh Jones: Metal-Organic Frameworks and Dr Ahmed Eissa: Biopolymers) towards the design and synthesis of novel host architectures (MOFs or biopolymers) that will then be employed to accommodate guest enzymes towards catalytic studies. Both MOF / Biopolymer surface adsorption as well as complete encapsulation of our target enzymes will be explored here. The target material described here will be characterised via numerous techniques such as XRD (powder and single crystal); SEM-EDX and TEM. All porous polymers will be assessed using (for instance) GC-MS and NMR studies.
In summary, the successful execution of this project would give rise to a novel family of heterogeneous host-guest enzyme catalysts. Furthermore, the candidate will glean vital experience in the fields of enzyme kinetics, coordination chemistry (MOF design, synthesis and characterisation) and biopolymer chemistry (e.g. porous polymer scaffolds).
References
1. (a) J. Mehta, N. Bhardwaj, S. K. Bhardwaj, K.-H. Kim and A. Deep. Coord. Chem. Rev. 2016, 322, 30-14. (b) X. Lian, Y. Fang, E. Joseph, Q. Wang, J. Li, S. Banerjee, C. Lollar, X. Wang and H.-C. Zhou. Chem. Soc. Rev., 2017, 46, 3386—3401. (c) S. Huang, X. Kou, J. Shen, G. Chen and G. Ouyang. Angew. Chem. Int. Ed. 2020, 59, 8786 –8798. (d) N. Ye, X. Kou, J. Shen, S. Huang, G. Chen and G. Ouyang. ChemBioChem 2020, 21, 2585–2590. (e) X. Gao, Q. Zhai, M. Hu, S. Li and Y. Jiang. Catal. Sci. Technol., 2021, 11, 2446–2455.
2. (a) K. M. L. Taylor-Pashow and J. G. Pribyl. 2019, 27, 1-26. (b) Muhammad Bilal and Hafiz M.N. Iqbal. Int. J. of Biological Macromolecules. 2019, 130, 462–482. (c) R. A. Wahab, N. Eliasa, F. Abdullaha and S. K. Ghoshal. Reactive and Functional Polymers. 2020, 152, 104613.
For more information: For an informal discussion please contact via direct email to Dr Ahmed Eissa (A.M.Eissa@wlv.ac.uk)
Supervisory Team: Dr Leigh Jones (SL, Chemistry) and Dr Ahmed Eissa (SL, Chemistry)
About the Project:
In 2016, Dr Leigh Jones and his co-workers discovered that the monometallic complexes of general formula: [Mn(III)F3(H2O)(L)] (where L = 1,2-diimine ligand; Fig 1a and 1b) were extremely easy to synthesise in high purity and good yields (>65%) in a short space of time (< 5 mins).1 More recent unpublished results have shown that these complexes are able to catalyse the oxidation of trans-stilbene and the sulfoxidation of 4-nitrothioanisole and 4-nitrophenyl phenyl sulphide in competitive yields (75-95%).2
![Crystal structures of [Mn(III)F3(H2O)(1,10-phen)] (a) and [Mn(III)F3(H2O)(2,2-bipy)] (b). (c) Schematic illustrating the potential binding sites within the MOF UiO-67-bipy.](https://cdn-wlvacuk.terminalfour.net/media/departments/faculty-of-science-and-engineering/images/Picture1.jpg)
Figure 1 Crystal structures of [Mn(III)F3(H2O)(1,10-phen)] (a) and [Mn(III)F3(H2O)(2,2-bipy)] (b). (c) Schematic illustrating the potential binding sites within the MOF UiO-67-bipy.
This project will focus on using Post-Synthetic Modification (PSMet) techniques) to coordinate fluoride bound transition metal centres (starting with Mn(III) and Fe(III); both of which are commercially available) into the empty diimine sites located within the Metal-Organic Framework (MOF) UiO-67-bipy (Fig. 1c). Upon successful production of these novel materials ([TMF2/3(sol)x-bpy-UiO]), their ability to catalyse various oxidations (e.g. trans-stilbene) will be probed.3 The structure of the precursor MOF UiO-67-bipy comprises {Zr(IV)6} metal cluster nodes connected via linear 2,2¢-bipyridine-5,5¢-dicarboxylate ligands to give a porous extended architecture (BET surface area = 2277 m2 / g; pore size = 7.2 Å) along with the required uncoordinated bipyridyl sites required for metal ingression (as propose here). Indeed, Manna and co-workers have shown how Ir and Pd centres can be successfully integrated into bpy-UiO (with only small reductions in pore size) towards forging highly efficient heterogeneous catalysts.4
The target MOF material will be characterised using a number of techniques. Powder XRD will ascertain whether integration was successful upon comparison with the spectrum of the UiO-67-bipy precursor. ICP-MS, EDX and XRF will be employed to analyse the degree of TM loading (Zr:TM ratio). The extent of metal (TM / Zr host metal node) leaching post-reaction(s) will be monitored using ICP-MS, while p-XRD will assess MOF stability post use.
In summary, the successful execution of this project would mean that manganese and iron fluorides salts (among others) were effective and commercially cheap starting materials (w.r.t. rare earth metals such as Pd) in the production of novel heterogeneous MOF materials. It is worth stating that a plausible alternative / additional project direction would be to carry out H2 gas storage assessments upon production of such F- rich MOFs (driven by strong H…F hydrogen bonding interactions) using our newly acquired gas adsorption isotherm technology.
References
1. E. Houton, B. Kelly, S. Sanz, E. J. L. McInnes, D. Collison, E. K. Brechin, A. G. Ryder, A.-L. Barra and L. F. Jones. Eur. J. Inorg. Chem., 2016, 32, 5123.
2. C. Ratanasakaprakan, P. J. Murphy, D. Keddie, L. F. Jones. Unpublished Results.
3. K. Hasan, N. Brown, C. M. Kozak. Green Chemistry. 2011, 13, 1230.
4. K. Manna, T. Zhang and W. Lin. J. Am. Chem. Soc. 2014, 136, 6566.
For more information: For an informal discussion please contact via direct email to Dr Leigh Jones (Leigh.Jones@wlv.ac.uk)


/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/Architecture-students-tree-planting.png)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/250630-SciFest-1-group-photo-resized-800x450.png)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/Andy-Lane-WLV.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/images-2024/Arthi-Arunasalam-teaser.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/submitted-news-images/Muslim-woman-playing-football.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/submitted-news-images/Business-School-800x450.jpg)
/prod01/wlvacuk/media/departments/digital-content-and-communications/submitted-news-images/University-of-the-Year.jpg)
